Abstract
Background: The majority of systemic peripheral T-cell lymphomas (PTCLs) have an aggressive behavior and compared to diffuse large B-cell lymphoma, survival is inferior using standard anthracycline-based treatment. Patient and disease factors impact survival, and the International Prognostic Index (IPI) is the most widely used clinical tool for prognostication for aggressive lymphomas. The IPI has shown prognostic utility in some PTCL entities such as systemic anaplastic large cell lymphoma (ALCL) and PTCL not otherwise specified (PTCL NOS), but less usefulness for others such as angioimmunoblastic T-cell lymphoma (AITL) as well as the rarer predominantly extranodal PTCL subtypes. T-cell specific prognostic indices such as the Prognostic Index for PTCL-U (PIT) have been explored but the IPI remains the most commonly used prognostic tool. The NCCN-IPI prognostic index has recently been developed for DLBCL. The NCCN-IPI builds on much the same parameters as the IPI, but with age stratified into four groups, lactate dehydrogenase (LDH) ratio using the upper limit of normal (ULN) separated into three categories (<1 ULN, 1-3 ULN, >3 ULN) and it also incorporates specific extranodal sites (CNS, gastrointestinal, lung or bone marrow involvement) but not absolute number of sites, with a maximum score of 8 points. In this study we evaluated the NCCN-IPI in comparison to the IPI and PIT in the three main nodal PTCL subgroups: ALK-neg ALCL, AITL, and PTCL NOS.
Methods: A cohort of newly diagnosed adult PTCL patients treated with anthracyline-based or other curative intent chemotherapy regimens was assembled from the Swedish Lymphoma Registry (SWE), University of Iowa/Mayo Clinic SPORE Molecular Epidemiology Resource (MER), and British Columbia Cancer Agency (BCCA). Patients with complete prognostic data and ALK-neg ALCL, AITL or PTCL NOS subtypes were included. Event-free survival (EFS) was defined as time from pathologic diagnosis to progression/relapse, retreatment, or death due to any cause. Kaplan-Meier curves and Cox proportional hazards models were used to examine prognostic utility of indices; concordance was measured via Harrell's c-index.
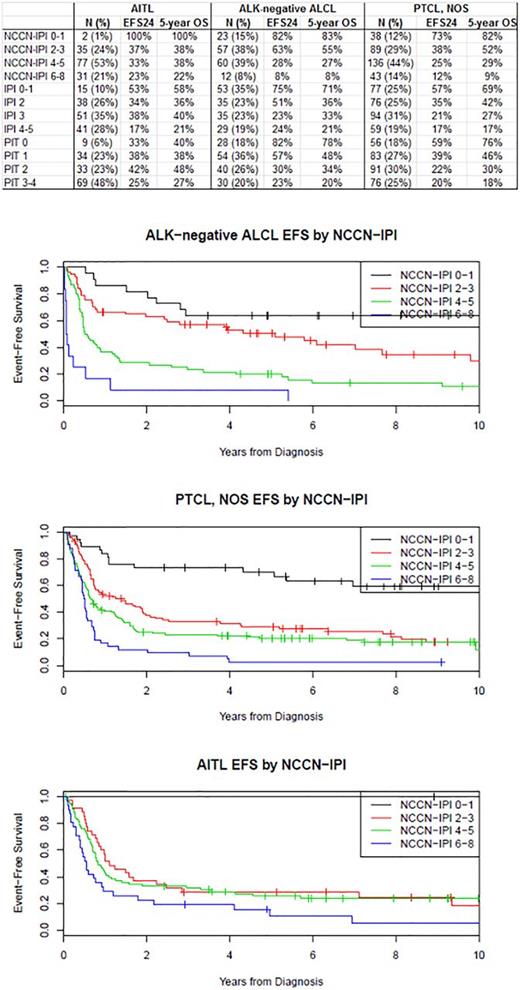
Results: In total, 603 patients were included. The majority of AITL presented with advanced stage disease (92%) and elevated LDH (75%) while ALK-neg ALCL patients less often exhibited adverse factors (advanced disease [67%] and elevated LDH [50%]) and PTCL NOS patients showed characteristics in between. At a median follow-up of 81 months (range 1-185), 449 patients (75%) had an event and 400 patients (66%) had died. Kaplan-Meier curves for EFS by NCCN-IPI are shown in figure 1. In patients with AITL (N=145), the NCCN-IPI had limited prognostic utility (EFS c-index=0.580) which was worse than the IPI (c-index=0.597) and similar to the PIT (c=0.585). The number of AITL patients with low-risk disease by NCCN-IPI was very small (N=2 [1%]). The NCCN-IPI had excellent prognostic utility in N=152 patients with ALK-neg ALCL (c-index=0.683), which was superior to the IPI and PIT (both c-index=0.665). However, a limited number of patients were classified as low risk (15%) or high risk disease (8%). The NCCN-IPI had modest prognostic utility in 306 patients with PTCL, NOS (c-index=0.629), which was inferior to the IPI (c-index=0.645) and similar to the PIT (c-index=0.631). Similar to ALK-neg ALCL, a limited number of patients were classified as low risk (12%) or high risk (14%) disease.
Conclusions: The NCCN-IPI performed similarly to the IPI with the exception of ALK-neg ALCL where it appeared to be better at defining low and very high risk patients. In AITL, the NCCN-IPI had very limited prognostic ability and performed even worse than the IPI (which also had limited prognostic ability). Better risk stratification is needed in this disease. For PTCL-NOS, the NCCN-IPI performed similarly to the IPI and PIT. While regrouping of scores could potentially improve this situation, the integration of other clinically defined prognostic factors and molecular characteristics is needed for more precise prognostic models in PTCLs.
Ellin: CTI: Consultancy; ROCHE: Consultancy, Research Funding. Connors: NanoString Technologies, Amgen, Bayer, BMS, Cephalon, Roche, Genentech, Janssen, Lilly, Merck, Seattle Genetics, Takeda,: Research Funding; Bristol-Myers Squibb: Research Funding; Amgen: Research Funding; Seattle Genetics: Research Funding; NanoString Technologies: Research Funding; Lilly: Research Funding; Merck: Research Funding; Cephalon: Research Funding; Genentech: Research Funding; Janssen: Research Funding; Takeda: Research Funding; F Hoffmann-La Roche: Research Funding; Bayer Healthcare: Research Funding. Smedby: Celgene: Membership on an entity's Board of Directors or advisory committees; Janssen: Research Funding. Ansell: Bristol-Myers Squibb: Research Funding; Celldex: Research Funding; Merck: Research Funding; Seattle Genetics: Research Funding; Affimed: Research Funding. Slack: Seattle Genetics: Consultancy. Cerhan: Janssen: Other: Scientific Advisory Board (REMICADELYM4001); Janssen: Other: Multiple Myeloma Registry Steering . Savage: Celgene: Consultancy; Roche: Research Funding; Seattle Genetics: Consultancy, Honoraria; Bristol-Myers Squibb: Honoraria; Merck: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.